Six Actions for Academic Health Systems to Achieve a High-Performing Clinical Trials Program
Federal government research funding cuts to Academic Health Systems (AHSs) are posing a threat to the academic mission of these organizations, and to their clinical trial programs. AHSs must find other sources of funding for clinical trials and they are looking to the pharmaceutical industry, but these companies are often frustrated with AHSs’ slow processes to open trials – on average over 8 months, and their lower than expected accrual.
The complex multi-unit structures of AHSs create special challenges for streamlining and improving clinical trial operations and they have not been subject to the same level of process improvement that is common in clinical and financial departments. During this time of financial crisis for research, it more important than ever for AHSs to expand private sector support for clinical trials and to:
- Optimize the efficiency of their clinical trial programs
- Develop plans for them to have a higher level of enrollment performance
- Be more financially sustainable
- Be more attractive to private sector partners
Below, we provide six ways to achieve these goals. More information on the current state and challenges of clinical trial programs in AHSs can be found in the appendix.
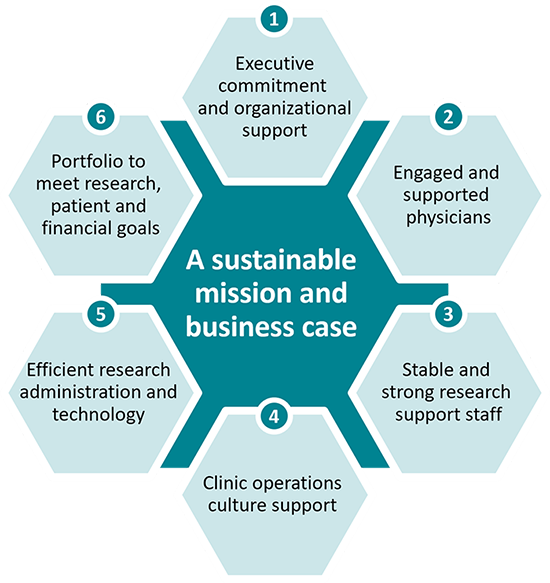
1. Engage executives for organizational support
Enterprise-wide executive involvement is needed to develop:
- A vision and goals for clinical trials consistent with the organization’s academic/research mission and clinical operations and financial goals;
- A common appreciation for the capabilities needed for achievement of the vision;
- An enterprise-wide strategic and business plan for clinical trials with a charge to implement with urgency;
- Clarity around decision rights and accountability to drive action.
The plan should address essential organizational support including information technology, human resources, legal, finance, etc. with a return on investment analysis.
2. Develop a plan to engage physicians
A virtually universal challenge is balancing pressures for clinical productivity (for both access and financial stability) with time and commitment to research and clinical trials. A plan to support physician time is essential, and it should include technology support and other ways to ease the burden.Success will require support from the faculty practice plan and department/division leadership with performance monitored.
3. Ensure strong and stable research support staff at the point of care
Staff turnover is costly; it undermines accrual and financial performance and is a source of dissatisfaction for physician investigators.Engage human resources for a plan to support recruitment and retention with strategies that include a career ladder, competitive compensation, and standardizing roles across the enterprise, ensuring work at the top of the license. For example, research nurse roles, regardless of where they work, should be standardized and reflect the need for a nursing credential.
4. Integrate clinical trials into clinical service line operations and the clinical practice culture
To be effective, the clinic operation must embrace the importance of clinical trials as part of clinical care delivery, enable a clinical practice culture that supports trials, and utilize tools to improve efficiency (e.g., clinical trial matching technology). Continuously remind clinic staff and leadership of the value of clinical trials for patients, for clinical programs, and for the academic enterprise. Reinforce the understanding that research is a key component of the strength of the AHS brand in the market and growth.
5. Ensure efficient research administration and processes
The benchmark time to open a clinical trial is 90 days or less. Meeting the benchmark requires enough of the right people with the right skills, support (e.g. technology, IT, finance) to perform efficiently, optimized technological solutions, and often re-engineered work processes.
Research functions are not core to health care operations. As an alternative, AHSs may consider “buy versus build” options as there are outsource partnership models for research administration functions that can accelerate improvement.
6. Offer a clinical trial portfolio that meets research, patient accrual and financial goals
A clinical trial’s portfolio (i.e. trial type, sponsor) is determined by the clinical departments/divisions and their investigators. As a result there is variation in program strength and performance. More than ever, it is important to have effective feasibility review, standardized processes, and benchmarks for departments/divisions to achieve strong research, accrual and financial performance.
Special attention to achieving clinical trial financial goals is needed. Investigator-initiated trials, many funded as part of federal grants, may require new sources of funding to maintain their vibrancy in the new political climate. Private sector sponsored treatment trials may be a larger proportion of the portfolio in the immediate years ahead for financial sustainability for clinical trial programs.
Next Steps
Given the reductions in research funding, strengthening the performance of clinical trials programs must be a priority. This requires a clear vision, an assessment, rigorous performance metrics and remedial intervention, including restructuring where needed. The six recommended actions offer a path for AHS leadership to follow so that the research mission of their organizations is sustained with other sources of funding, and that vital research continues to advance discovery and be available to patients.
Appendix:
Current State and Challenges for Clinical Trials Programs in Academic Health Systems
Many of the functions supporting clinical trials are managed in different organizational units that include a medical school, health system, faculty practice plan, and at times university-level administration–each with different mission critical and financial priorities. Across clinical departments/divisions, there are variations in scientific strength, operational efficiency, staffing levels, and accrual and financial performance targets for trials.
Efficiency and high performance requires the support of all operating units and an enterprise-wide plan that includes process improvement, stakeholder commitments, and regular oversight of performance to goals and applying the same process improvement rigor that is deployed within clinical and financial operations. Pharmaceutical companies want to work with organizations that have efficient processes and strong accrual—strong accrual supports the research mission of the AHS.
In our work with AHSs, we consistently encounter a number of common challenges:
- Organization – Absence of overarching and explicit clinical trial program goals aligned with enterprise-wide research, clinical operations, and financial goals;
- Operations – Lack of rigorous research workflow and financial optimization review. Multiple organizational units complicate clarity and accountability. Bottlenecks are common with long times to open new studies—an average of 8.1 months for hospitals;
- Enrollment – Frequent failure to meet trial specific accrual targets with one survey reporting average cancer trial accrual of 1.5 patients;
- Financial Performance – Decentralized and underperforming financial management processes (i.e. trial budgeting, revenue cycle) that result in lost or unrealized revenue.
Improving clinical trial operations requires broad organizational support and executive engagement.
AAMC Leads The impact of federal actions on academic medicine and the U.S. health care system. June 14, 2025. . Accessed August 7, 2025
Woolfe S, Galea S, Williams D. The potential impact of the Trump administration policies on health research in the USA. The Lancet, Volume 405, Issue 10495, 2114-2116. June 14, 2025
Association of Academic Medical Centers AAMC news. What's at stake when clinical trials research gets cut. April 24, 2025. . Accessed August 8, 2025.
Smith S. Accelerating Clinical Trial Activation, Applied Clinical Trials. June 21, 2024. Accessed July 15m 2025https://www.appliedclinicaltrialsonline.com/view/accelerating-clinical-trial-activation
O’Brien D, Sher R, Morin A, Achieving a High-performing, Diverse Accrual Clinical Trial Program in an Academic Health System. Manatt Whitepaper, May 2024.
Ryan DP, Chief's Perspective on Physician Burnout. JCO Oncol Pract 20, 11-13 (2024). DOI:
Goldhaber N, Jacobs MB, Laurent L, Knight R, Zhu W, Pham D, Tran A, Patel S, Hogarth M, Longhurst C, Integrating clinical research into electronic health record workflows to support a learning health system, JAMIA Open, Volume 7, Issue 2, July 2024, ooae023, .
Fritter J, Jones C. Mapping the Pathway to Take Control of Your Clinical Research Career. Clin Res (Alex). 2023 Oct;37(5):7-15. Epub 2023 Oct 17. PMID: 38873692; PMCID: PMC11172395.
Georgieff T, Clinical Trial Matching Solutions: Understanding the Landscape Applied Clinical Trials Feb 28, 2024.
Hennessey M, New Trends and Tactics in Outsourcing. Applied Clinical Trials. 33 (10) Oct 15, 2024. . Accessed June 14, 2025.
O’Brien D. The Real Challenges that Stop Hospitals from Integrating Clinical Trials into Clinical Care. Editorial Clinical Research as a Care Option web site. May 13,2024 https://theconferenceforum.org/editorial/the-real-challenges-that-stop-hospitals-from-integrating-clinical-trials-into-clinical-care
Lee C, Werner TL, Deal AM, Krise-Confair CJ, Bentz TA, Cummings TM, Grant SC, Lee AB, Moehle J, Moffett K, Peck H, Williamson S, Zafirovski A, Shaw K, Hofacker JK. Clinical Trial Metrics: The Complexity of Conducting Clinical Trials in North American Cancer Centers. JCO Oncol Pract. 2021 Jan;17(1): e77-e93. doi: 10.1200/OP.20.00501. Epub 2020 Nov 13. PMID: 33186085; PMCID: PMC8202063.
