HHS Outlines Plans for $100 Billion Provider Relief Fund
Following weeks of uncertainty, the Department of Health and Human Services (HHS) on Wednesday, April 22, released additional details about its plans to distribute direct relief funds to offset providers’ expenses and revenue losses due to the COVID-19 pandemic. The Coronavirus Aid, Relief, and Economic Security (CARES) Act, Public Law 116-136, signed into law on March 27, included $100 billion in Public Health and Social Services Emergency Fund (referred to as the “Provider Relief Fund”) appropriations to be distributed by grants or other payment mechanisms to healthcare providers. For more information on the law, see the Manatt Insights summary.
On April 10, HHS released a “first tranche” of funding—$30 billion from the Provider Relief Fund—to providers who received Medicare fee-for-service (FFS) claims payments in calendar year 2019. (For more information, see Manatt Health’s analysis.) Yesterday’s announcement provides details about how the remaining funding will be distributed. The announcement came following the Senate’s passage of an additional $75 billion for the same fund as part of the Paycheck Protection Program and Health Care Enhancement Act (PPPHCEA, H.R. 266, also referred to as “CARES Act 3.5”). The House has now passed CARES Act 3.5 and the President is expected to sign the bill shortly; HHS’s announcement does not address how the newly enacted $75 billion in funding would be distributed among providers.
Overview
The CARES Act left significant discretion to the Secretary of HHS related to both distribution of the Provider Relief Fund and definition of eligible providers.1 The legislation also specifies a wide range of permissible uses for the Provider Relief Fund, including compensation for lost revenue; building or construction of temporary structures; leasing of properties, medical supplies, and equipment, including personal protective equipment and testing supplies; increased workforce and trainings; emergency operation centers; retrofitting facilities; and surge capacity. The legislation says only that HHS must distribute the funding “in consideration of the most efficient payment systems practicable to provide emergency payment.” In recognizing the imperative to deliver funding to providers quickly, the discretion that Congress provided to the agency, and the competing demands for the funding, HHS has elected to release funds in “tranches” or waves, which has resulted in a delay in the distribution for some providers.
Figure 1. Timeline of Provider Relief Fund Legislative and Executive Actions to Date
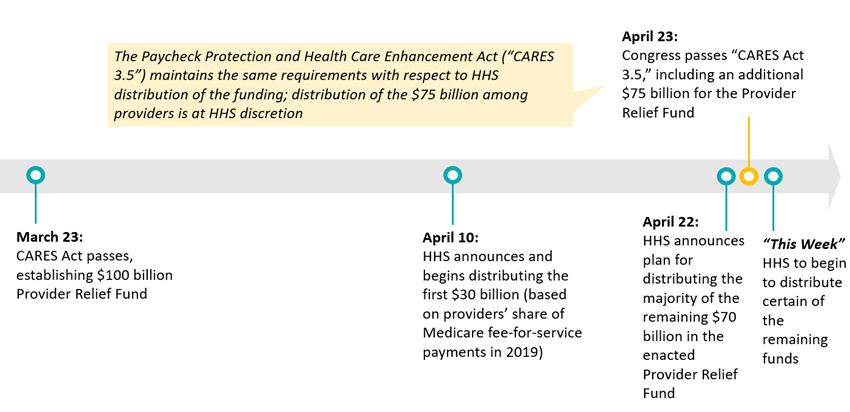
Figure 2. $100 Billion Provider Relief Fund Distribution Methodology (Announced April 22)

Specific Allocations
$20 Billion Additional “General Allocation” to Begin on Friday
HHS announced that $20 billion will be released under a “general distribution” methodology to Medicare facilities and providers, adding to the $30 billion distributed earlier this month. Accordingly, a total of $50 billion—half of the original $100 billion—will be allocated proportionately based on eligible providers’ 2018 net patient revenue.
- HHS’s initial $30 billion allocation was distributed proportionate to providers’ share of 2019 Medicare fee-for-service reimbursements and was essentially a “down payment” on the amount owed under the general allocation.
- Beginning Friday, April 24, and continuing on a rolling weekly basis, HHS will distribute the additional $20 billion based on the revenue data providers submit in CMS cost reports (for applicable providers) or via revenue information submitted through a new portal that will open this week (accessible at https://www.hhs.gov/providerrelief).
- Note that providers, such as acute care hospitals, that automatically receive additional stimulus deposits from this fund will still be required to submit 2018 revenue information through the web portal for verification even after receipt of funds.
As with the first distribution, providers will need to sign an attestation confirming receipt of the funding and agreeing to HHS’s Terms and Conditions, including a prohibition on balance billing for presumptive or actual COVID-19 patients (see below).
HHS did not offer further insights into its methodology to calculate the proportionate share distributions. However, this round of disbursements will take into account payers other than Medicare FFS, such as Medicare Advantage, commercial payers, and Medicaid. Under this methodology, providers with higher overall revenues (e.g., providers with relatively larger numbers of higher reimbursing commercial patients) and those with a lower share of Medicare FFS revenues will receive more funding.
$10 Billion Allocated to “High Impact” Areas or “Hot Spots”
HHS will allocate $10 billion to hospitals in areas that have been particularly impacted by COVID-19, so-called “hot spots.” This funding will be in addition to any share these providers receive from the general allocation pool. Of the $10 billion pool, New York will reportedly receive approximately $4 billion.
By 3:00 pm EST on April 27,2 hospitals are required to submit to HHS information about their total number of intensive care unit beds as of April 10, 2020, and the total number of admissions with a positive diagnosis for COVID-19 from January 1 through April 10. HHS will use this information to target distribution of the dollars.
HHS’s announcement also indicates that it will take into account the additional challenges that hospitals serving a disproportionate number of low-income patients face (relying on their Medicare Disproportionate Share Hospital (DSH) adjustment).
$10 Billion Allocated to Rural Providers
Another $10 billion is designated for rural health clinics and hospitals. HHS does not provide detail about this tranche of the funding, other than to say that it will be distributed “using a methodology that distributes payments proportionately to each facility and clinic” and taking into account operating expenses. The funding will be distributed as early as the week of April 27. This funding will be in addition to any share the providers receive from the general allocation pool.
$400 Million Allocated to Indian Health Service (IHS)
This funding will be distributed based on IHS facility operating expenses as early as next week.
Use of the Provider Relief Fund to Cover COVID-19 Treatment for the Uninsured
On Friday, April 3, HHS Secretary Alex Azar announced during the Administration’s daily press briefing that the agency would implement a program to directly reimburse hospitals for COVID-19-related care of uninsured patients using monies from the Provider Relief Fund. Today’s announcement includes additional detail about this tranche of funding, without establishing a set amount of funding that will be used for this purpose.
The Health Resources and Services Administration (HRSA) will use a claims-based system to distribute both the CARES Act Provider Relief Fund funding (for testing and treatment) and $1 billion appropriated in the Families First Coronavirus Relief Act (P.L. 116-127) to reimburse providers for uninsured testing. Claims for services rendered on or after February 4 will be paid at Medicare rates using the current year Medicare fee schedule and will be available to cover:
- Specimen collection, diagnostic, and antibody testing.
- Testing-related visits, including in the following settings: office, urgent care or emergency room, or via telehealth.
- Treatment: office visit (including via telehealth); emergency room; inpatient; outpatient/observation; skilled nursing facility; long-term acute care (LTAC); acute inpatient rehab; home health; durable medical equipment (DME, e.g., oxygen, ventilator); emergency ground ambulance transportation; non-emergent patient transfers via ground ambulance; and Food and Drug Administration (FDA)-approved drugs, as they become available for COVID-19 treatment, administered as part of an inpatient stay.
- When an FDA-approved vaccine becomes available, it will also be covered.
- For inpatient claims, date of admittance must be on or after February 4, 2020.
As a condition of receiving reimbursement under the HRSA program, providers must attest to several conditions, including that they have checked for other health coverage to confirm that a patient is uninsured and that they will not balance bill the patient. HRSA is expected to issue guidance about the program over the coming weeks; the agency’s guidance about how providers can satisfy the eligibility check requirement, particularly for claims predating the start date of this program, will determine how feasible it will be for providers to benefit from this program.
Hospitals and other healthcare providers who provided testing or treatment for uninsured COVID-19 patients on or after February 4 can submit claims for reimbursement once they have registered and completed training for the new program. The provider registration portal will become available to providers on April 27; HHS anticipates that providers will be able to begin submitting claims on May 6 and that reimbursement will be available beginning mid-May.
Additional Allocations to Be Determined
Finally, the HHS announced that some of the remaining funding will be reserved for skilled nursing facilities, dentists, and providers that solely rely on Medicaid because the above-described methodologies would not otherwise account for their lost revenues. HHS did not provide information about either the amount or the methodology for this distribution.
Open Questions Remain for Medicaid Providers
The amount available to be distributed through the “additional allocations” described above is unclear. With approximately $70.5 billion accounted for through the specific allocations announced yesterday and an unspecified—but likely sizable—portion of the $100 billion CARES Act Fund allocated to reimburse providers for uncompensated care costs, it seems likely little will remain from the first $100 billion to fund these “additional allocations.”
Because the largest portion of the funding will be distributed based on overall revenues, providers with higher reimbursement rates will fare better than others. Since Medicaid generally reimburses less than other payers, providers with high Medicaid volumes are likely to receive a lower share of funding under the HHS distribution method, yet these providers are likely to be facing the greatest challenges from lost revenues. This is true for high-volume Medicaid hospital providers as well as a range of other community providers.
While some safety net providers (which often treat high volumes of Medicaid-enrolled and uninsured patients) will be able to seek reimbursement for uninsured costs through the HRSA-administered testing and treatment fund, that funding only supports COVID-19 testing and treatment, and not loss of revenues. Behavioral health providers, substance use providers, and many home health providers are seeing declining revenues during this period, and yet HHS’s distribution methodology disadvantages them relative to providers with greater Medicare or commercial revenues. In many parts of the country, Medicaid is also the largest payer for pediatric care and skilled nursing facilities. While the “additional allocations” described above may help fill some of these gaps, the distribution methodology described here is likely to amplify requests from providers to target a portion of the new $75 billion in CARES Act 3.5 funding to the most vulnerable providers.
Terms and Conditions for Accepting Funding
As with the first tranche of funding, providers who receive funds through the direct provider reimbursement allocations described above are required to agree to a set of Terms and Conditions specified by HHS or to return the money if they elect not to adhere to them within 30 days of receipt. (A different process will apply to the HRSA-administered uninsured claims described above.) The Terms and Conditions include prohibitions on certain applications of the funding, prohibit recipients from using the funding for expenses that have been or will be otherwise reimbursed, and prohibit providers from “balance billing” patients. The Terms and Conditions for the newly allocated $20 billion distribution also require providers to consent to public disclosure by HHS of the award amount.
HHS stated in its guidance related to the initial $30 billion distribution, “These are payments, not loans, to healthcare providers, and will not need to be repaid.” The Terms and Conditions specify that all providers must retain cost documentation and other information “required by future program instructions to substantiate the reimbursement of costs.” CMS also states on the Provider Relief Fund website that “there will be significant anti-fraud and auditing work done by HHS, including the work of the Office of the Inspector General.”
Providers receiving over $150,000 are subject to further CARES Act reporting requirements, including submitting a report no later than 10 days after the end of each calendar quarter on uses or obligations of funding, detailed descriptions of projects or activities using large portions of funds, related job creation (if any), and details on use of subcontractors.
HHS has established a portal to facilitate delivery of the Provider Relief Fund. Providers will report their revenues through this portal; even providers who received automatic distributions based on their cost reports will use the portal to verify revenue information. Providers will also use a portal to attest to receipt of funding, to indicate their acceptance of the Terms and Conditions, or to initiate a process to return the funds.
Supplemental Appropriations for the Provider Relief Fund Expected
The legislative language in the CARES Act 3.5 is identical to the provision in the CARES Act, meaning HHS will determine the use of the funds. It could do so in a fashion akin to its distribution of the first $100 billion or take an altogether different approach.
1 Eligible providers in the CARES Act extend beyond acute care hospitals. This statute defines “eligible health care providers’’ as “public entities, Medicare or Medicaid enrolled suppliers and providers, and such for-profit entities and not-for-profit entities not otherwise described in this proviso as the Secretary may specify, within the United States (including territories), that provide diagnoses, testing, or care for individuals with possible or actual cases of COVID–19.
2 HHS extended the deadline on April 23.
