Achieving Clinical Trial Diversity: Expanding Hospital Capacity to Offer Trials in the Community
Editor’s Note: In our recent white paper, Achieving Clinical Trial Diversity: Expanding Hospital Capacity to Offer Trials in the Community, Manatt Health discusses the important role of community hospitals and health systems in expanding access to clinical trials for diverse populations in the community, shares how to leverage hospitals’ ability to contribute to advancing science by supporting research related to the trials, and offers strategies to advance these efforts. Based on the guidance provided in the paper, coauthor Donna O’Brien was invited to give a presentation at the National Academies of Sciences, Engineering and Medicine’s (NASEM) Forum on Drug Discovery, Development and Translation on October 12. The presentation, summarized below, focused on how to expand community hospital capacity to offer clinical trials and engage with diverse populations. Manatt is hosting a free webinar on November 2 that will provide a deep dive into the issue of increasing community hospitals’ capacity to support clinical trials. Click here to learn more and register. Even if you can’t attend the live program, register now and receive a link to view the webinar on demand.
The inclusion of racial and ethnic groups in clinical trials has been a national priority for decades, but progress toward that end has been limited. When the Covid-19 pandemic threw into stark relief the underlying inequities in health care access (and thus in access to clinical trials), Congress and the Administration were moved to action. In parallel, trial sponsors, hospitals, and other stakeholders have been accelerating efforts to increase diverse populations’ access to clinical trials. Given the complexity of the challenge, much more action is needed at multiple levels and by all involved.
Access to clinical trials depends upon how easily a patient can access care generally, whether providers offering the appropriate trial for the patient are nearby, whether the patient has trust in the physician recommending the clinical trial, and whether and at what level the patient has health care coverage to pay for care. These conditions often are not met, resulting, even under the best circumstances, in the exclusion of people of color and lower-income patients. For most people, including diverse populations, the point-of-care is in a community hospital or their outpatient programs or physician practices. Though there are examples of robust clinical trial programs being offered by community hospitals, many do not offer trials—or their capacity to do so is limited. Examples of initiatives to expand trials in the community to reach diverse populations include a program launched by the National Cancer Institute in the 1980s1 and recent efforts by pharma companies2, 3 and advocacy groups,4 but the scale of these efforts and funding are limited. There remains significant untapped potential for expansion of trials in the community.
To make progress, it is crucial to understand the health care ecosystem within which clinical trials are offered. Many initiatives aimed at breaking down barriers to access focus on the critical need to build trust between providers and diverse populations in the community. Of equal importance is the need to strengthen the overall capacity of the health care delivery system infrastructure to support clinical trials at the point of care.
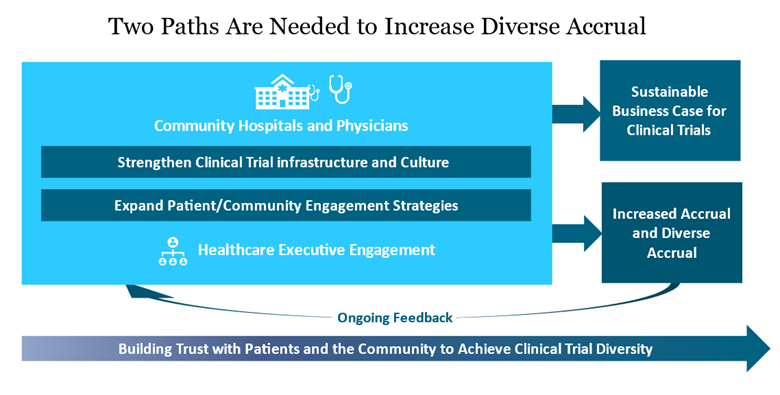
In Manatt’s recent white paper, we provide more context on the factors that influence access to clinical trials, including insurance coverage related barriers, and offer concrete strategies that can be used by community hospitals and trial sponsors, which include the pharmaceutical industry, the National Institutes of Health (NIH), academic medical centers (AMCs) and others. Strengthening hospital capacity to offer trials is a complex undertaking. The engagement of hospital and health system executives by trial sponsors and others is central, both to enhancing strategies to engage with diverse populations and patients and to addressing the challenges of infrastructure and the clinical practice culture, as well as for supporting the development of a mission and business case for expansion of clinical trials to reach diverse populations in the community. The figure below shows the parallel paths to be pursued.
Specific Actions for Community Hospitals and Trial Sponsors
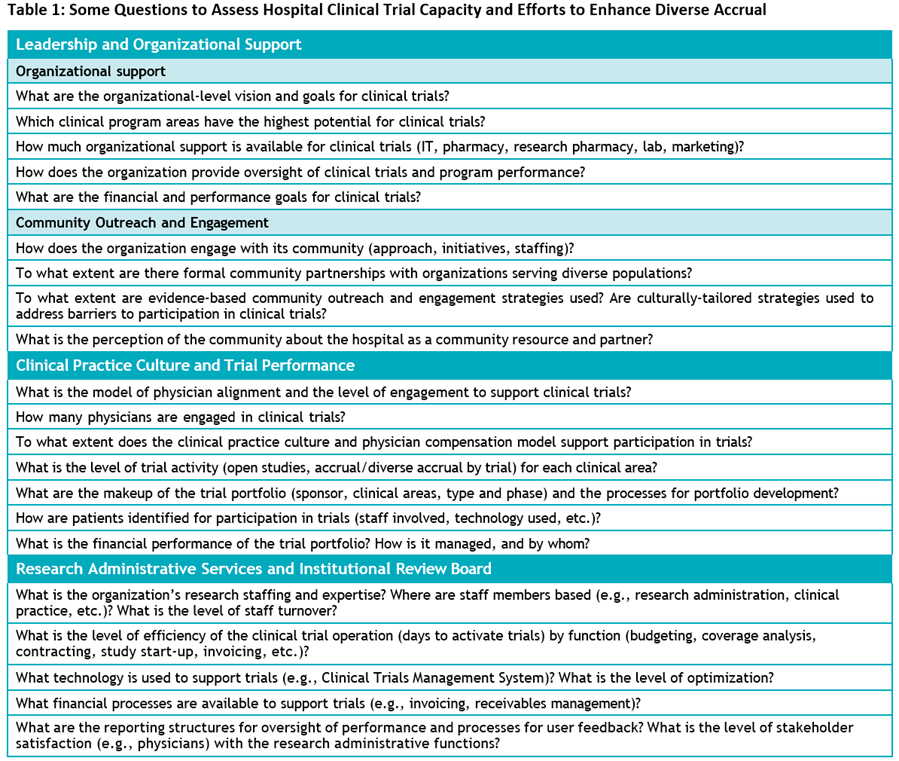
In Manatt’s white paper, we present two case studies—one from an integrated delivery system and one from the NCI Community Cancer Centers Program.5, 6 Both are focused on building capacity with executive-level support. We provide self-assessment questions, noted in Table 1, and recommend these specific actions for community hospitals and trial sponsors to consider:
Proposed Actions for Community Hospital Providers
- Assess current clinical trial activity and the supporting infrastructure across the organization (see Table 1, which lists assessment categories and questions).
- Identify opportunities for expansion of clinical trials, based on clinical program strengths (e.g., cancer, cardiology, neurology) and determine the resources and actions required.
- Develop a sustainable plan with a business and operational strategy for expansion of clinical trials.
- Learn more about outsourcing and partnership models to inform a build/buy decision on research administrative services and clinical trial operations.
- Reach out to trial sponsors to discuss collaborative opportunities and to build a business case to expand capacity and accrual to trials.
- Approach payers to propose a value strategy to support expansion of clinical trials (e.g., grants and/or enhanced reimbursement for patients on clinical trials), so funds can be used, following best practices for capacity-building.
- Link clinical trial outreach to hospital programs for community outreach and engagement.
- Identify opportunities to expand use of evidence-based practices to engage with diverse populations and incorporate culturally tailored strategies to build trust and address barriers to accrual.
Proposed Actions for Trial Sponsors (pharma, NIH, AMCs)
- Identify high-potential but underperforming community hospital sites for clinical trials, and work with executives to assess those hospitals’ organizational and clinical culture barriers to effective accrual and diverse accrual (see Table 1).
- Work with these hospitals’ executive leadership to support development of a business case for their clinical trial program.
- Engage executives of community hospitals to develop and fund disease-specific, best-practice-sharing hospital consortia that serve diverse populations and to provide expertise for building or expanding support functions.
- Develop an evaluation approach for hospitals to assess the factors that influence diverse accrual to trials to help inform their program investment and a sustainable business case.
Only by bringing robust clinical trials to local communities and their diverse populations—and offering them through trusted high-quality providers—can accrual to trials be significantly expanded, leading to improved treatments and better health for all.
1 National Cancer Institute. The Community Clinical Oncology Program and the Minority-Based Community Clinical Oncology Program: Accomplishments in Cancer Clinical Trials. Bethesda, MD: NCI; 2011. Accessed Aug. 3, 2023.
2 Genentech website. Public statement. Genentech launches oncology clinical trial diversity alliance. June 23, 2021.Accessed Aug. 3, 2023.
3 PhRMA website. Press release. PhRMA joins top academic leaders to announce new community-based initiative to enhance clinical trial diversity. July 19, 2022. Accessed Aug. 3, 2023.
4 American Heart Association website. News release. Awardees named for $20 million project to foster diversity in clinical trial research. May 17, 2022. Accessed Aug. 3, 2023.
5 Kaluzny A, O’Brien D. Managing Disruptive Change in Healthcare: Lessons from a Public-Private Partnership to Advance Cancer Care and Research. New York, NY: Oxford University Press; 2015.
6 Dimond EP, St Germain D, Nacpil LM, et al. Creating a "culture of research" in a community hospital: Strategies and tools from the National Cancer Institute Community Cancer Centers Program. Clin Trials. 2015 Jun;12(3):246-56. doi: 10.1177/1740774515571141. Epub 2015 Feb 17. PMID: 25691600; PMCID: PMC4420772.